Earle H. Spaulding recognised a disinfection framework was required since all reusable medical devices cannot be sterilized1
The Spaulding Classification met a key unmet need that today still forms the basis of international medical device disinfection guidelines.
The Spaulding Classification
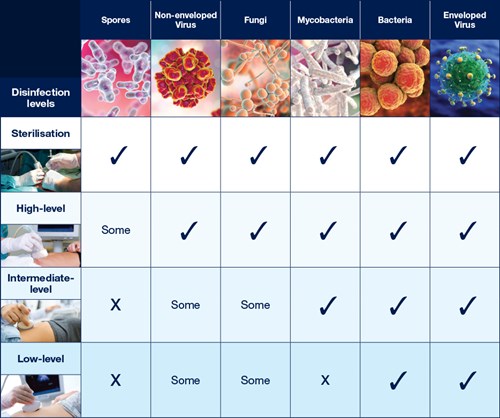
The Spaulding Classification stratifies the risk of infection transmission based on the patient tissue the device will contact during use. The device classification determines the level of disinfection/sterilization required (Table 1).
Table 1: Overview of The Spaulding Classification.1-4
|
Spaulding Classification |
Medical Device Contacts |
Risk of Infection Transmission |
Disinfection Level |
|
Critical |
Sterile tissue or the bloodstream |
High |
Sterilization* |
|
Semi-critical |
Mucous membranes or non-intact skin |
Medium |
High Level Disinfection (HLD) |
|
Non-critical |
Intact skin only |
Low |
Intermediate level (ILD) or Low level disinfection (LLD) |
* Critical ultrasound probes can be high level disinfected and used with a sterile sheath if sterilization is not possible.2
Sterilization
Sterilization is a validated process used to render a product free from viable micro-organisms.2
Critical devices must be sterile when used.1
High Level Disinfection (HLD)
HLD is a process that kills vegetative bacteria, mycobacteria, fungi, lipid and nonlipid viruses, as well as some, but not necessarily high numbers of, bacterial spores.2,3
Semi-critical ultrasound probes must undergo HLD and be used with a sheath.2
Critical ultrasound probes that cannot be sterilized can also undergo HLD.2 They must also be used with a sterile sheath.
Low & Intermediate Level Disinfection
An intermediate level or low level instrument grade disinfectant may be used for disinfection of non-critical instruments.2
Low level disinfectants are capable of killing most vegetative bacteria and some fungi, as well as enveloped (lipid) viruses. They do not kill mycobacteria, non-enveloped viruses, or bacterial spores.2
Intermediate level disinfection is a process capable of killing vegetative bacteria, mycobacteria, fungi, and lipid and nonlipid viruses.2
Understanding Disinfection
It is important to recognize there are differences between disinfection levels and sterilization. Correctly applying The Spaulding Classification to medical devices is a key part of keeping patients safe from HAIs.
References
1. Spaulding EH (1968). Chemical disinfection of medical and surgical materials. Disinfection, sterilization, and preservation. Lawrence C, Block SS. Philadelphia (PA), Lea & Febiger: 517-531.
2. Canadian Standards Association (CSA). CAN/CSA-Z314-18 Canadian medical device reprocessing. 2018.
3. Health Canada. Guidance Document: Safety and Effectiveness Requirements for High-Level Disinfectants and Sterilants for use on Reusable Semi-Critical and Critical Medical Devices. 2018.
4. Health Canada. Guidance Document: Information to Be Provided by Manufacturers for the
Reprocessing and Sterilization of Reusable Medical Devices. 2011
